- Findings were published from Dassault Systèmes’ five-year collaboration with the FDA in response to needs for faster, safer medical device evaluation
- The peer-reviewed report serves as a guide for establishing credibility in medical device in silico clinical trials
- Virtual twins that accurately simulate specific aspects of patient populations allow for the refinement, reduction and replacement of human and animal testing in clinical trials
Dassault Systèmes Collaboration Yields Breakthrough Guide for Using Virtual Twins in Clinical Trials
Dassault Systèmes Press Contacts
Corporate / France
Arnaud MALHERBE
arnaud.malherbe@3ds.com
+33 (0)1 61 62 87 73
North America
Natasha LEVANTI
natasha.levanti@3ds.com
+1 (508) 449 8097
EMEA
Virginie BLINDENBERG
virginie.blindenberg@3ds.com
+33 (0) 1 61 62 84 21
China
Grace MU
grace.mu@3ds.com
+86 10 6536 2288
Japan
Reina YAMAGUCHI
reina.yamaguchi@3ds.com
+81 90 9325 2545
Korea
Jeemin JEONG
jeemin.jeong@3ds.com
+82 2 3271 6653
Dassault Systèmes (Euronext Paris: FR0014003TT8, DSY.PA) today announced the availability of the world’s first guide for the medical device industry that outlines how to use virtual twins to accelerate clinical trials. This guide was published following the successful completion of a five-year collaboration with the U.S. Food and Drug Administration. The in silico clinical trial “ENRICHMENT Playbook” marks a significant advancement in the integration of virtual twins into the regulatory process in response to needs for improved patient safety, regulatory compliance, and pace of innovation.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20241029374860/en/
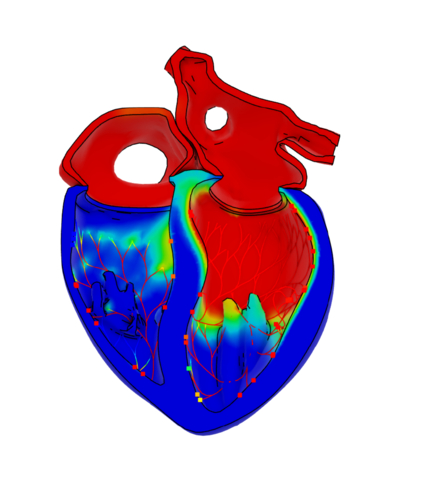
A cross-section of the heart highlighting the electrical activity around 220 milliseconds into the cardiac cycle, with specific regions in the left ventricle among the first to become active. Image: Dassault Systèmes
The peer-reviewed, open-access 44-page publication serves as a comprehensive guide for establishing credibility in medical device in silico clinical trials by providing a hierarchical framework and strategic approach to overcome challenges. The project involved the creation of virtual twins that simulate patient populations with unprecedented accuracy – an innovative approach that allows for the refinement, reduction and replacement of human and animal testing. Industry can access knowledge on running trials, building patient populations, and interpreting and measuring results to better understand the effects of a device before it reaches a patient.
“As the healthcare industry continues to embrace digital transformation, the ENRICHMENT Playbook represents a pivotal moment in the evolution of clinical trial methodologies,” said Claire Biot, Vice President, Life Sciences and Healthcare Industry, Dassault Systèmes. “The strong participation and input from diverse experts was crucial in ensuring that the playbook meets the highest scientific and regulatory standards and is highly practical for immediate application in the field. We remain committed to driving collaborative innovation in this sector to improve the patient experience.”
The ENRICHMENT Playbook outlines the credibility assessment process based on recommendations from the recently issued FDA final guidance Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions, and appears in the CDRH/OSEL Regulatory Science Tools Catalog. It is the result of extensive collaboration among leaders from the FDA’s Office of Science and Engineering Laboratories, clinical practice, academia, the medical device industry and regulatory science. The playbook’s authors are discussing outcomes and opportunities today at Dassault Systèmes’ International Virtual Human Twin Experience Symposium in Paris.
###
FOR MORE INFORMATION
The ENRICHMENT Playbook: https://discover.3ds.com/fda-enrichment-clinical-trial
Dassault Systèmes’ 3DEXPERIENCE platform, 3D design software, 3D Digital Mock Up and Product Lifecycle Management (PLM) solutions: http://www.3ds.com
SHARE THIS ON THE SOCIAL PLATFORM X
Five-Year @Dassault3DS and FDA collaboration yields breakthrough guide for virtual patient twin-enriched medical device clinical trials
Connect with Dassault Systèmes on
https://x.com/Dassault3DS
https://www.facebook.com/DassaultSystemes
https://www.linkedin.com/company/dassaultsystemes
https://www.youtube.com/DassaultSystemes
ABOUT DASSAULT SYSTÈMES
Dassault Systèmes is a catalyst for human progress. We provide business and people with collaborative virtual environments to imagine sustainable innovations. By creating virtual twin experiences of the real world with our 3DEXPERIENCE platform and applications, our customers can redefine the creation, production and life-cycle-management processes of their offer and thus have a meaningful impact to make the world more sustainable. The beauty of the Experience Economy is that it is a human-centered economy for the benefit of all – consumers, patients and citizens. Dassault Systèmes brings value to more than 350,000 customers of all sizes, in all industries, in more than 150 countries. For more information, visit www.3ds.com
© Dassault Systèmes. All rights reserved. 3DEXPERIENCE, the 3DS logo, the Compass icon, IFWE, 3DEXCITE, 3DVIA, BIOVIA, CATIA, CENTRIC PLM, DELMIA, ENOVIA, GEOVIA, MEDIDATA, NETVIBES, OUTSCALE, SIMULIA and SOLIDWORKS are commercial trademarks or registered trademarks of Dassault Systèmes, a European company (Societas Europaea) incorporated under French law, and registered with the Versailles trade and companies registry under number 322 306 440, or its subsidiaries in the United States and/or other countries. All other trademarks are owned by their respective owners. Use of any Dassault Systèmes or its subsidiaries trademarks is subject to their express written approval.
View source version on businesswire.com: https://www.businesswire.com/news/home/20241029374860/en/
 Business wire
Business wire 











Add Comment